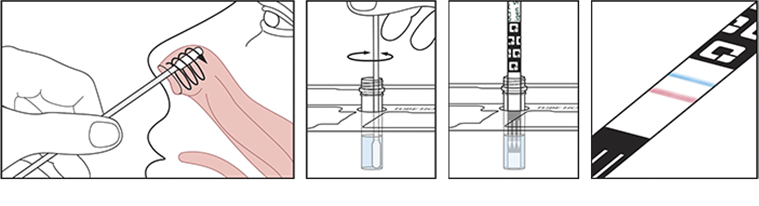
QuickVue At-Home OTC COVID-19 Test lets you get rapid results, in the privacy of your own home. Available over-the-counter, everything you need is in the package and taking the test is simple.
This home test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older. The test to be used in individuals within 6-days of symptom onset or individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.