For Professional Use Only
COVID-19 Testing Options
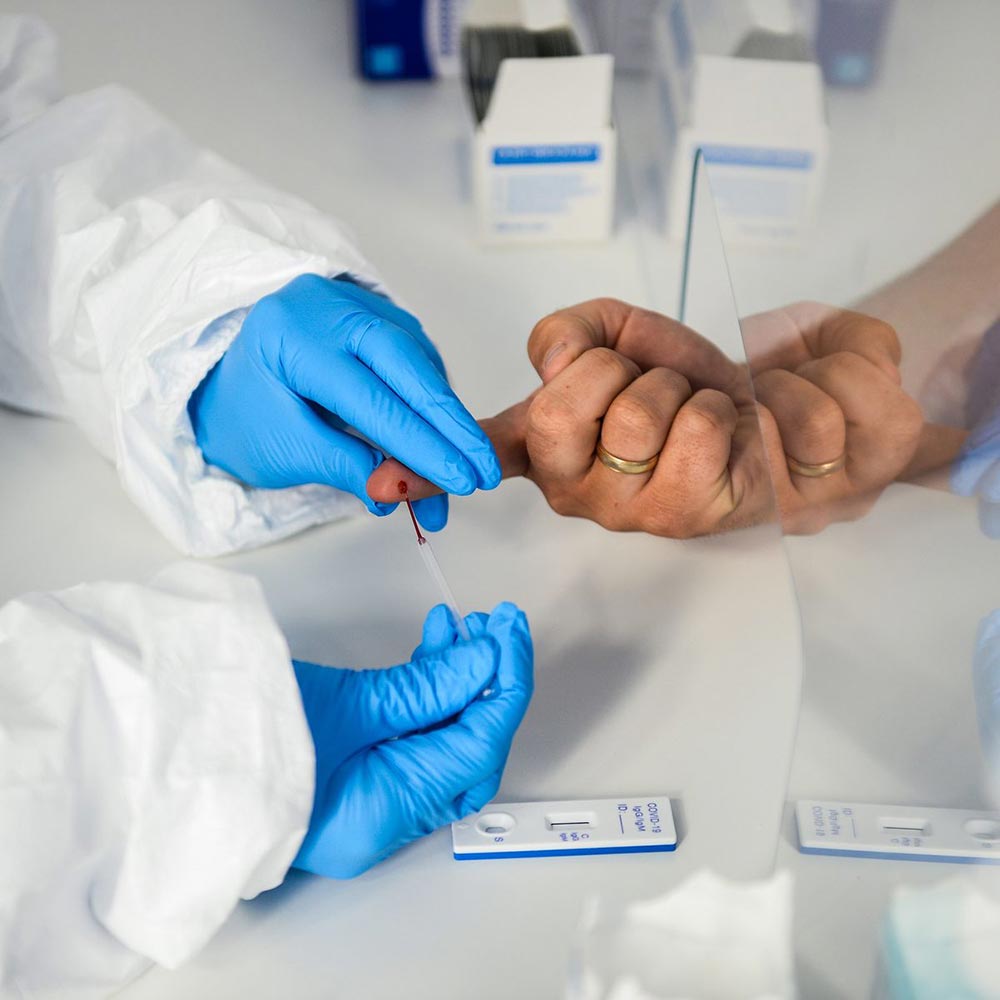
Rapid Antibody Serology IgM & IgG Fingerstick
Results in 15 minutes.
The test has been authorized by the FDA under an EUA for use by authorized laboratories. We are affiliated with a moderate/high complexity lab. The lab interprets the results for validation. Antibody tests are looking for previous exposure to the Covid-19 virus greater than seven days. It will not show an active infection with a positive or negative result.
*The sensitivity of the test itself to IgM is 87.9% and specificity is 100%
*The sensitivity of the test to IgG is 97.2% and specificity is 100%.
Rapid RT PCR Nasal
The COVID-19 RT-PCR test is a real-time reverse transcription-polymerase chain reaction (RT-PCR) test for the qualitative detection of nucleic acid from SARS-CoV-2 in upper and lower respiratory specimens (such as nasopharyngeal) collected from individuals suspected of COVID-19 by their healthcare provider.
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable in respiratory specimens during the acute phase of infection.
*The sensitivity of the test is about 98%
*The specificity of the test is about 100%
Rapid Antigen Test
Results in 15 minutes.
The rapid COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal swab specimens directly collected from individuals who are suspected of COVID-19 by their healthcare provider within five days of symptom onset.
This test has not been FDA cleared or approved; this test has been authorized by FDA under a EUA for use by authorized laboratories; use by laboratories certified under the CLIA, 42 U.S.C. §263a, that meet requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
*The sensitivity of the test is about 88.4%
*The specificity of the test is about 100%
Rapid Covid-19/Flu A&B
Visually read in 15 minutes.
Sensitivity and Specificity
− COVID-19 – Sensitivity 93.9%, Specificity 100%
− Flu A – Sensitivity 91.4%, Specificity 95.7%
− Flu B – Sensitivity 87.6%, Specificity 95.9%
FDA Emergency Use Authorization (EUA)
Visually read in 15 minutes
Flocked nasopharyngeal swab for superior specimen collection and patient comfort
Insite Onsite

What has Covid-19 taught us? It has taught us that we need to be able to react effectively to challenges that affect our personnel and business. The only thing that is for sure during the next six months is uncertainty. New questions, research, and advice are produced every day that will challenge your business.
Where do you go when you have questions and need answers? Most businesses do not have the time or expertise to interpret the medical issues surrounding our current pandemic. That is why we are here to assist as your Medical Leadership Team in helping you and your team achieve success in these chaotic times. Your business needs to perform today, not wait for answers until tomorrow.
Access to multiple Medical Doctors for guidance and leadership. Helping your team to maintain productivity and decrease liability. For more information please contact us to learn more.
FAQ
Employers can ask employees to provide the following information:
- A positive result for, or other diagnosis with, COVID-19;
- Symptoms of infection with COVID-19, e.g., fever of or over 100.4°F, cough, shortness of breath, sore throat;
- “Close contact” (as defined by the Centers for Disease Control) with any person who has tested positive for, or has otherwise been diagnosed with, COVID-19 infection within the preceding 14 days;
- Whether the employee has been asked to self-quarantine by a health official within the preceding 14 days;
- Whether the employee has traveled to, or stopped over in, a country for which the CDC has issued a Level 3 travel health notice; and
- Depending on geographic location, whether the employee is considered “high risk” for COVID-19 infection, meaning over age 60, pregnant, or suffering from diabetes, lung disease, heart disease, asthma, HIV, or similar conditions.
Employers may be able to require employees to be tested if they have symptoms of COVID-19 and, nonetheless, assert that they are fit for work.
Can employers take employees’ temperature before permitting them to enter the employer’s facilities?
Yes. However, employers should implement a temperature check protocol to ensure that temperature checks are designed to reduce the threat that an employee with COVID-19 poses to the workplace. Temperature checks should be reliable, effective, performed consistently, and respect employees’ privacy. For example, all employees entering facilities should be checked only by trained personnel and the results should be treated as confidential.
Generally, no. HIPAA imposes obligations to safeguard protected health information (PHI) only on covered entities, which are defined to include health plans, health care clearinghouses, and health care providers. An employer acting in its capacity as an employer is not subject to HIPAA. Other laws, such as the Americans with Disabilities Act (ADA) or state confidentiality laws, may apply.
No. The ADA prohibits such a disclosure. However, the employer can provide co-workers with information that would help them evaluate the risk of infection.
Covid-19 health care costs are covered by health insurance carriers. A doctor’s visit is necessary for the claim to be filed and paid. The POC test (Nasal or oral swap) is covered. Rapid antibody tests are currently covered if they are performed by a CLIA (certified lab) testing facility. Rapid antibody testing is used for a mass pre-screening and is not diagnostic or giving medical advise. The test is only intended for informational purpose and is the participant’s responsibility to seek medical care pending test outcome.
Fill out the form below to submit a request to purchase COVID-19 test kits. Test kits will be fulfilled on a first-come, first-serve basis based on supplies available.
